Background/Radiobiology
Radiation is energy that travels through space and matter. It is of two types: electromagnetic (EM) and particulate (Table 1). EM radiation is massless and moves through a vacuum at 3×108 m/s. It can be ionising or non-ionising. Particulate radiation is energy in the form of subatomic particles [1]
| Electromagnetic Radiation | Particulate Radiation | ||
|---|---|---|---|
In order of increasing frequency:
|
Sub-Atomic Particle | Elementary Charge | Relative Atomic Mass |
| Alpha (He24) — directly ionising | +2 | 4 | |
| Proton (H1) — directly ionising | +1 | 1 | |
| Neutron (n0) — indirectly ionising | 0 | 1 | |
| Electron (e-/β-) — directly ionising | -1 | 0.0005 | |
| Positron (e+/β+) — directly ionising | +1 | 0.0005 | |
| Table 1: Examples of particulate radiation. The charge and relative atomic mass of particulate radiation is also given [1,11]. | |||
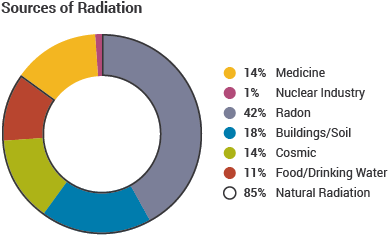
Directly ionising radiation strips electrons from atoms electrostatically. Indirectly ionising radiation causes electrons to be ejected from their atom by Compton scatter and the photoelectric effect. In the former, the fraction of the photon energy transferred to an outer electron is proportional to the cosine of the scatter angle. In the latter, all the photon energy is transferred to an inner electron. An electron must acquire an energy greater than its binding energy to be ejected [1]. The common sources of background radiation are given in Figure 1. The average exposure per year, per individual, is ≈2.4 mSv, which is equivalent to a fatal cancer risk of 0.012% for individuals aged between 30-60 [2,3]
Terminology in nuclear medicine
| Radiation | LET (keV/μm) |
|---|---|
| 1.25 MeV Co60 γ-ray [5] | 0.25 |
| 250 kVp x-rays [6] | 2 |
| 10 MeV proton [6] | 5.7 |
| 20 keV β-particle [7] | 10 |
| 1 keV electrons [6] | 12.3 |
| 5 MeV neutron [7] | 20 |
| 5 MeV α particle [7] | 50 |
| Table 2: Linear energy transfer for a range of radiation types and energies. Alpha particles have the highest LET, and hence are most potent when inside the body, due to their low velocity and high mass. | |
Linear energy transfer (LET) describes how much energy (keV) a radiation-beam transfers to its surroundings per metre (Table 2). The LET of radiation increases with charge and mass, and decreases with kinetic energy [1]. The higher the LET, the more biologically potent the radiation. The LET for particulate radiation increases as it loses energy whilst traversing a medium [4].
Relative biological effectiveness (RBE) is the ratio of the dose of radiation of type x, Rx required to produce the same biological effect as a reference dose RR which is normally a high-energy x-ray beam (250 kVp) or a gamma-ray from Cobalt-60;
RBE = Rx ⁄ RR for a given effect (1)
RBE increases with LET until a critical point of ≈100 keV/μm. The overkill region arises because the increasing dose has no further biological effect [1].
The RBE of radiation may vary depending on its subcellular distribution: for example, when situated outside the cell, Auger electrons have an RBE of ≈1; this value increases ≈10 - fold, however, when localised within the nucleus [8].
Radiation Units
| Radiation type | Radiation weighting factor |
|---|---|
| X-rays | 1 |
| Gamma-rays | 1 |
| Electrons | 1 |
| Positrons | 1 |
| Protons > 2 MeV | 2 |
| Alpha particles | 20 |
The radiation-absorbed dose is the total energy absorbed by tissue. It is given in rad or Gray (Gy), 0.01 Gy = 1 rad. The equivalent dose is the absorbed dose multiplied by the radiation weighting factor, WR and is given in roentgen-Equivalent-Man (rem) or Sievert (Sv), 0.01 Sv = 1 rem. The effective dose, E (Eq-2) is the sum of the tissue-weighted equivalent dose, WT multiplied by the equivalent dose, HT for all tissue types, T. It arises because the same equivalent dose of radiation has different biological effects on different tissues [1].
E = ∑T ( HT × WT ) (2)
Biological Effects
The biological effects of ionising radiation are caused by the secondary electrons from an ionisation event, not the primary radiation beam [9]. These electrons deposit energy within tissues causing molecular damage and the formation of toxic chemical species. Although ionisation and free-radical production occur on a sub-second time scale, biological effects may take years to manifest. [10].
Free radicals such as hydroxyl (OH•) and hydrogen (H•) form when radiation interacts with water. Secondary and tertiary molecules including (O2•-) and hydrogen peroxide (H2O2) are then produced, and interact with endogenous nitrogen molecules such as nitric oxide (NO•) to produce reactive nitrogen species including nitrogen dioxide (NO2•) and peroxynitrite (ONOO-). These molecules may cause DNA damage, protein oxidation or lipid damage [2]. Biological effects are classified as stochastic or deterministic. Stochastic effects are probabilistic: the likelihood of them developing increases with radiation dose. They are primarily caused by low-radiation doses and have no lower-bound threshold. Deterministic effects are dose-dependent: the severity of the biological effects increases with radiation dose; they have a lower-bound threshold and are primarily caused by high-dose radiation [1,11].
Biological Range
Different types of radiation have different ranges in tissue (Table 3). Short-range radiation is used therapeutically to target localised lesions as it transfers its’ energy to surrounding cells more effectively than long-range radiation. Long-range radiation is used in medical imaging. Short-range radiation is more biologically potent.
| Radiation type | Range in tissue |
|---|---|
| Auger electrons | 0.02-10 μm [12] |
| Alpha | 10-100 μm [13] |
| Beta | few mm-few cm [14] |
| Gamma | Many cm [14] |
| X-ray | |
| Neutron | |
| Table 3 - Range of radiation in tissue. Radiation with a large mass, high charge and/or low energy have the shortest range. | |
Direct and Indirect Biological Effects
Direct effects, following ionisation or atomic excitation, include the breakage of molecular bonds of DNA (deoxyribonucleic acid) or proteins, molecular degradation and intermolecular cross-linking. They occur within a picosecond of radiation exposure and are typically induced by high-LET radiation [2,15]. Indirect effects, due to low-LET radiation, occur over a longer period and are mediated by free radicals and reactive oxygen or nitrogen species [15]. The majority of DNA damage occurs due to indirect effects because water, the source of free radicals, contributes 70% of cellular composition [11]. However, direct DNA damage is more potent because radiation with a high-LET can induce multi-strand breaks [16]. Indirect effects may occur at a distance from the initial radiation site. In addition to DNA strand-breakages, base losses or changes also occur. Non-irradiated cells may express radiation-induced biological effects secondary to the release of signals from directly-irradiated cells in their vicinity. This is termed the bystander effect and is distinct from the abscopal effect in which tumour cells distant from the primary radiation-site diminish in size. The latter is thought to be mediated by the immune system [17]. Although the number of DNA lesions is large for a given radiation dose (1000 single-strand breaks and 40 double-strand breaks per Gy [10]), the number of cell fatalities is low: most radiation-induced DNA changes are detected and repaired by enzymes. Persistent mutations can cause genetic and somatic effects, as well as cell death The consequence of an un-repaired mutation will depend, in part, on the biological function of the affected gene [10].
Cellular Radiosensitivity
The radiosensitivity of a cell depends on several factors. First undifferentiated cells (those lacking a specific physiological role) are more radiosensitive than differentiated ones as the former give rise to the latter (Figure 2). Second, the stage of the cell cycle (G1, S, G2 and M): cells are least radiosensitive during the DNA replication (S-)phase because a large number of DNA repair molecules are present, and most radiosensitive during the (M)mitotic-stage. Third is the size of the nucleus: cells with larger nuclei are more radiosensitive. Fourth is the rate of the cell-cycle: cells that replicate more frequently are more radiosensitive as radiation-induced DNA damage is more likely to persist [11,18].
On a subcellular level, different organelles have different radiosensitivities: for example, both the cell membrane and nucleus are more radiosensitive than the cytoplasm [9,19].
The effect of radiation on cells can be visualised on a cell-survival curve, which plots the proportion of cells that survive at a particular absorbed dose of radiation (Figure 3).
The steepness of Figure 3b will increase in response to several factors including an elevated local cellular oxygen concentrations (as oxygen stabilises free radicals, prolonging their half-life) and radiation with a higher LET. The oxygen-enhancement ratio (OER) is the ratio of the radiation dose required to produce a particular biological effect in hypoxic cells, CH and oxygenated cells, CO; OER = CH/CO. Its value is ≈3 for low-LET radiation and ≈1 for high-LET radiation [10].
Chronic and Acute Effects
Biological effects may be acute or chronic. Chronic effects arise following multiple low-dose radiation exposure events, primarily in slowly proliferating cells. The effects can be stochastic or deterministic and may manifest genetically (in future generations) or somatically (e.g: teratogenesis, reduced life expectancy); examples of deterministic effects along with their associated threshold-doses are given in Table 4. Stochastic effects include germ-cell mutations, and cancer: the likelihood of developing leukaemia or a solid cancer per 100 mSv is 1% [20].
| Biological effect | Chronic exposure | Total accumulated exposure threshold |
|---|---|---|
| Permanent sterility | 2-5 rad/week | 250-300 rad |
| Cataract | -------- | 400 rad (over 2 months) |
| Radiation dermatitis | 1-2 rad/day | 2000 rad |
| Table 4 – Deterministic effects following a chronic exposure to radiation. For the effects to manifest, the accumulated dose over time must be, at least, equal to the threshold value given in the far-right column | ||
Data is obtained from: [1]
Acute effects arise shortly after exposure to a high radiation dose, primarily in rapidly proliferating cells, and include erythema, conjunctivitis and acute radiation sickness (ARS). Table 5 gives the acute radiation dose required to produce several different biological effects. ARS has a natural history that is divided into 4 stages (Fig.10). In the prodromal stage, non-specific symptoms such as nausea and vomiting arise. The latent phase may last several weeks. Haemopoetic symptoms such as prolonged coagulation time and a dampened immune response arise at 250-500 rad. Gastrointestinal effects including gut ulceration and loss of intestinal villi occur at 500-1000 rad. Neurovascular symptoms include motor- and sensory-dysfunction, and reduced levels of consciousness develop at 5000-10,000 rad. Mild symptoms of ARS include fatigue, loss of appetite and sweating [11,21]. Figure 4 illustrates the consequences of, and relationship between the different components of ARS.
Chronic exposures, per unit of radiation, are less biologically significant than acute exposures as the body is capable of repairing any damage incurred between exposure events [22].
| Biological effect | Acute threshold radiation dose |
|---|---|
| Generalised erythema (skin reddening) | 200-600 rad |
| Temporary hair loss | 300-600 rad |
| Temporary sterility | 50 rad |
| Permanent sterility | 200-1000 rad (this effect is age dependent) |
| Cataract formation | 200-700 rad |
| Vomiting 2 hours post-exposure | 100-400 rad |
| Diarrhoea 1 hour post-exposure | 600-800 rad |
| Headache 4 hours post-exposure | 600-800 rad |
| Fever 1 hour post-exposure | 400-600 rad |
| Table 5 – The threshold acute radiation doses for a variety of biological effects.
The biological effects highlighted in blue arise during the prodromal stage of ARS following an acute radiation threshold dose given in the right-hand column. | |
Data is obtained from: [1]
References
- Bushberg JT, Seibert JA, Leidholdt EM, Boone JM. The Essential Physics of Medical Imaging. Lippincott Williams & Wilkins; 2011.
- Reisz JA, Bansal N, Qian J, Zhao W, Furdui CM. Effects of ionizing radiation on biological molecules--mechanisms of damage and emerging methods of detection. Antioxid Redox Signal 2014;21:260–92. Permalink: dx.doi.org/10.1089/ars.2013.5489
- ICRP. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP 2007;37:1–332. Permalink: dx.doi.org/10.1016/j.icrp.2007.11.001
- Laney T, Kooy H. Proton and Charged Particle Radiotherapy. Lippincott Williams & Wilkins; 2008. Permalink: dx.doi.org/10.1118/1.2907963
- V C on the BE of IRB, Sciences C on L, Studies D on E and L, Council NR. Health Effects of Exposure to Low Levels of Ionizing Radiation:: BEIR V. National Academies; 1990. Permalink: dx.doi.org/10.2307/3577873
- International Atomic Energy Agency. Radiation Biology: A Handbook for Teachers and Students. IAEA; 2010. Accessible online at: http://www-pub.iaea.org/books/IAEABooks/8219/Radiation-Biology-A-Handbook-for-Teachers-and-Students
- Dendy PP, Heaton B. Physics for Diagnostic Radiology, Third Edition. CRC Press; 1999.
- Howell RW, Narra VR, Sastry KS, Rao D V. On the equivalent dose for Auger electron emitters. Radiat Res 1993;134:71–8. Permalink: dx.doi.org/10.2307/3578503
- Mayles P, Nahum A, Rosenwald J. Handbook of Radiotherapy Physics: Theory and Practice. vol. 8. CRC Press; 2007. Permalink: dx.doi.org/10.1201/9781420012026
- Bailey D, Humm J, Todd-Pokropek A, Van-Aswegen A. Nuclear Medicine Physics: A Handbook for Teachers and Students. Vienna: International Atomic Energy Agency; 2014. Accessible online at: http://www-pub.iaea.org/books/iaeabooks/10368/Nuclear-Medicine-Physics
- Saha GB. Physics and Radiobiology of Nuclear Medicine. New York, NY: Springer New York; 2006.
- Welch MJ, Redvanly CS. Handbook of Radiopharmaceuticals: Radiochemistry and Applications. John Wiley & Sons; 2003.
- Hoskin PJ. Radiotherapy in Practice - Radioisotope Therapy. OUP Oxford; 2007. Permalink: dx.doi.org/10.1093/med/9780198568421.001.0001
- Australian Government - Department of Health. Ionising Radiation and Human Health 2012. http://www.health.gov.au/internet/publications/publishing.nsf/Content/ohp-radiological-toc~ohp-radiological-05-ionising (accessed November 22, 2015).
- Desouky O, Ding N, Zhou G. Targeted and non-targeted effects of ionizing radiation. J Radiat Res Appl Sci 2015;8:247–54. Permalink: dx.doi.org/10.1016/j.jrras.2015.03.003
- Powsner RA, Powsner ER. Essential Nuclear Medicine Physics. John Wiley & Sons; 2008. Permalink: dx.doi.org/10.1002/9780470752890
- Multhoff G, Pockley A, Gaipl U, Rodel F. Radiation-induced effects and the immune system. Frontiers E-books; 2013. Permalink: dx.doi.org/10.3389/fonc.2013.00055
- Bergonié J, Tribondeau L. Interpretation of Some Results of Radiotherapy and an Attempt at Determining a Logical Technique of Treatment / De Quelques Resultats de la Radiotherapie et Essai de Fixation d’une Technique Rationnelle. Radiat Res 1959;11:587–8. Permalink: dx.doi.org/10.2307/3570812
- Pouget J-P, Santoro L, Raymond L, Chouin N, Bardiès M, Bascoul-Mollevi C, et al. Cell membrane is a more sensitive target than cytoplasm to dense ionization produced by auger electrons. Radiat Res 2008;170:192–200. Permalink: dx.doi.org/10.1667/RR1359.1
- Committee to Assess Health Risks from Exposure to Low Levels of Ionising radiation. Health Risks from Exposure to Low Levels of Ionizing Radiation:: BEIR VII Phase 2. National Academies Press; 2006.
- Campeau F, Fleitz J. Limited Radiography. Cengage Learning; 2009.
- Langland OE, Langlais RP, Preece JW. Principles of Dental Imaging. Lippincott Williams & Wilkins; 2002.
- World Nuclear Organisation. Nuclear Radiation and Health Effects 2015. http://www.world-nuclear.org/info/Safety-and-Security/Radiation-and-Health/Nuclear-Radiation-and-Health-Effects/ (accessed November 14, 2015).